Environment & Energy
Related: About this forumDissolution Rates of Uranium Dioxide in Used Nuclear Fuel in an H2O2/Carbonate System Are Enhanced by Radiation.
The paper to which I'll refer in this post is this one: Study on the Dissolution Behavior of UO2 in the Na2CO3–H2O2 System Using a Flow-Through Dissolution Device Meng Zhang, Haofan Fang, Mingjian He, Caishan Jiao, Hongtao Zhao, and Chaobo Shang Industrial & Engineering Chemistry Research 2024 63 (50), 21662-21671.
The paper refers to a new, rather mild conditions (compared to the widely used PUREX, and other solvent separation methods depending on nitric acid) for the reprocessing of used nuclear fuel to recover its valuable components. This is the Carbex Process, which reduces the number of organic phase extractions to separate plutonium and uranium as compared to PUREX and the necessity of convoluted oxidation and reduction procedures, and avoids as well, the use of nitric acid to dissolve the used fuel pellets, substituting, hydrogen peroxide, H2O2 and sodium carbonate, Na2CO3.
It is generally employed after a technique called valoxidation, which involves heating the used fuel pellets to high temperatures in the presence of air or oxygen to drive off tritium (as tritiated water), cesium, rubidium, tellurium and under some conditions technetium and other elements with volatile oxides. This process also oxidizes uranium from the +4 valence state in UO2, uranium dioxide to U3O8, potentially either a mixed oxidation state, two U(V) and one U(VI) or a molecular orbital with distributed charge arrayed over three uranium atoms. (cf. Jayangani I. Ranasinghe, Linu Malakkal, Ericmoore Jossou, Barbara Szpunar, Jerzy A. Szpunar, Comprehensive study on the electronic and optical properties of (alpha)-U3O8, Computational Materials Science, Volume 171, 2020, 109264).
Recently in this space, there was an absurd claim, by an advocate of rebranding fossil fuels as "hydrogen," - a very dangerous prospect - that because a minor journal in the Springer Nature family of Scientific Journals (communications earth & environment) published a paper about a putative "hydrogen economy," a line of bullshit that is now 50 years old and still useless and ignorantly embraced, that therefore the following statement is true: "According to Nature.com, hydrogen is considered a crucial element for achieving net-zero greenhouse gas emissions." One hears these things and doesn't want to believe it. One sees these sorts of statements and one painfully understands how we have ended up in this awful political and physical mess. Stupidity rules, but is probably not worth dignifying these sorts of remarks by responding to them.
My point in including the previous paragraph is to remark that because I am discussing this process, I am not necessarily endorsing it. I just think it worthy of discussion. Overall, for the essential process of nuclear fuel reprocessing critical to human survival, but only slowly being recognized as such and quite possibly fitting into the rubric "too little, too late," I'm a fluoride volatilization kind of guy, which can handle the fuels better recovering valuable fission products as well as actinides, with the possible exception of making the recovery of valuable tritium more difficult, and possibly resulting in the release small amounts into the environment. (All nuclear plants do so, in trivial amounts.)
Tritium has the mass psychological property of producing orgies of fear and ignorance, the ever popular employment and celebration of stupidity, even though the amount of tritium in the environment has been falling from a huge peak in 1963, the era of nuclear weapons testing, before the majority of the time that the essential commercial nuclear industry was growing and operating. If you were alive in 1963 or born then or afterwards, you have been living with declining levels of environmental tritium for much of, or all of, your life, and look, tritium hasn't killed you, although something else will eventually. The cost of tritium today is roughly $30,000 per gram, with the main industrial uses being as a tracer, often in medical as well as environmental settings, and providing "glow in dark" watches, displacing the historical use of radium in this setting. It is also potentially useful for fusion reactors if in fact they ever become viable. It is said that one year's use of the experimental ITER reactor will consume the current world supply without providing any exergy (useful work) at all.
All this said, I applaud this paper, even as I don't endorse it, and I concede that the chemistry involved may indeed, under certain circumstances be industrially valuable. I especially admire the statement that used nuclear fuel is not, and should not be regarded as "waste." It's pretty damned valuable, particularly because it can accelerate access to fissionable materials beyond uranium, notably plutonium, but also americium, neptunium, and curium.
From the introduction to the paper:
The only commercially operated spent nuclear fuel reprocessing process now is the PUREX process. The PUREX process has been refined since its proposal, but unresolved issues remain, (4-8) including high costs and significant waste generation; strong oxidizing properties of nitric acid corrode equipment and necessitate extra safety measures. In response to these issues, researchers suggest using a relatively mild carbonate solution as a medium to replace concentrated nitric acid for dissolving SNF and separating uranium, plutonium, and other elements. The CARBEX process proposed in 2008 (CARBonate EXtraction) is one of the representatives. (9) The CARBEX process uses Na2CO3–H2O2 as the dissolution solution and utilizes actinide elements to form stable complexes with carbonate anions for selective extraction of actinide elements. (5,10-12) It is a promising spent fuel reprocessing process, but there is little research on the dissolution stage in the CARBEX process, especially on the dissolution behavior of spent fuel in the Na2CO3–H2O2 system...
The authors note that in proposed and actual "nuclear waste" dumps, a naturally occurring CARBEX type process may take place, not that such a process, to my mind, involves much real risk, but since antinukes - the drivers of the extreme global heating now observed - have shit for brains understanding of risk and rely on paranoia driven by selective attention, worrying that a radioactive atom might show up in their otherwise useless brains.
From the introduction:
The only commercially operated spent nuclear fuel reprocessing process now is the PUREX process. The PUREX process has been refined since its proposal, but unresolved issues remain, (4-8) including high costs and significant waste generation; strong oxidizing properties of nitric acid corrode equipment and necessitate extra safety measures. In response to these issues, researchers suggest using a relatively mild carbonate solution as a medium to replace concentrated nitric acid for dissolving SNF and separating uranium, plutonium, and other elements. The CARBEX process proposed in 2008 (CARBonate EXtraction) is one of the representatives. (9) The CARBEX process uses Na2CO3–H2O2 as the dissolution solution and utilizes actinide elements to form stable complexes with carbonate anions for selective extraction of actinide elements. (5,10-12) It is a promising spent fuel reprocessing process, but there is little research on the dissolution stage in the CARBEX process, especially on the dissolution behavior of spent fuel in the Na2CO3–H2O2 system.
It is probably best to tell the story found in the paper in some equations and graphics:
Water is subject to radiolysis, for good and for bad:
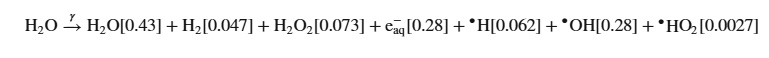
Mechanistically these free radicals interact to make products.

(No, the production of hydrogen is not an industrial route for this much hyped gas, the "G values" which represent the reaction rates per unit of radiation is small.)
In its +6 oxidation state, uranium forms a soluble carbonate complex, which the key to the entire process.

The authors note that this process in a so called "nuclear waste" dump - I oppose all waste dumps as I'm a closed cycle kind of guy - this process may take place, releasing some actinides.
The CARBEX process utilizes the solubility of U+6 to dissolve UO2 by oxidizing it with hydrogen peroxide:
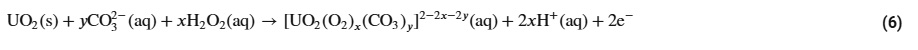 .
.
Some graphics:
The schematic for the experimental apparatus:
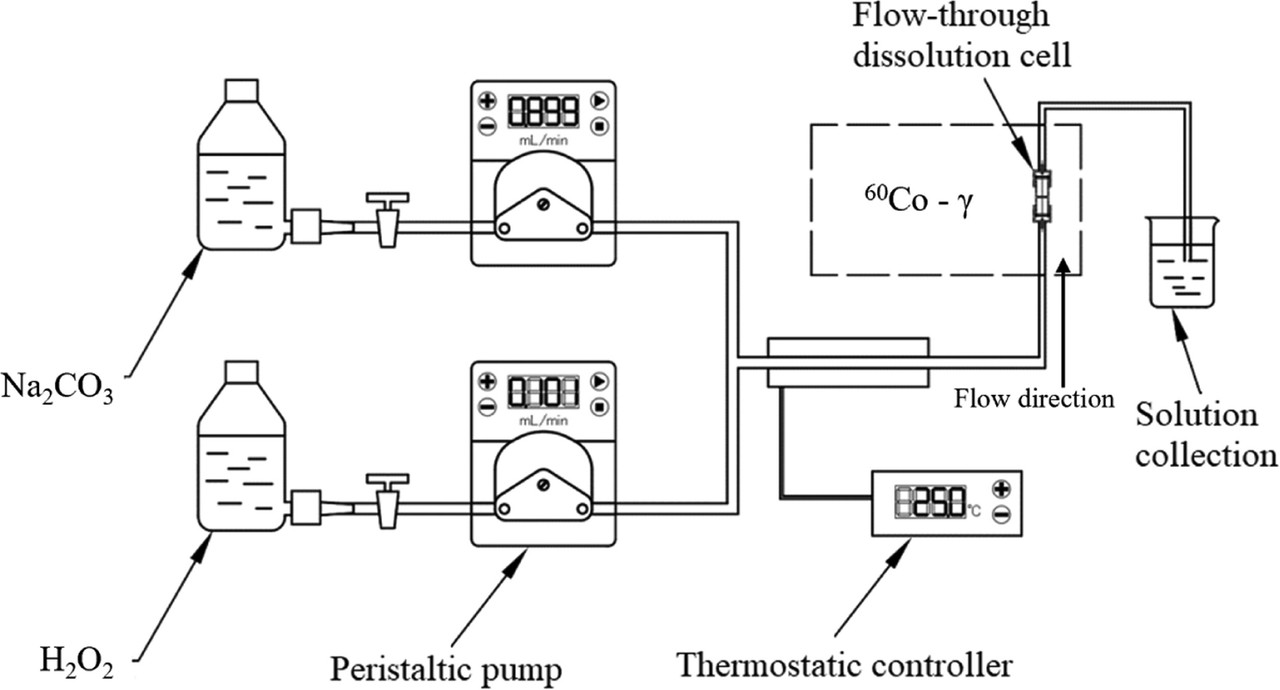
The caption:
The purpose of the paper is to show how radiation effects the hydrogen peroxide/carbonate system, and reaction rates. The authors evaluate the effects of varying parameters in the system as the following graphics will show:
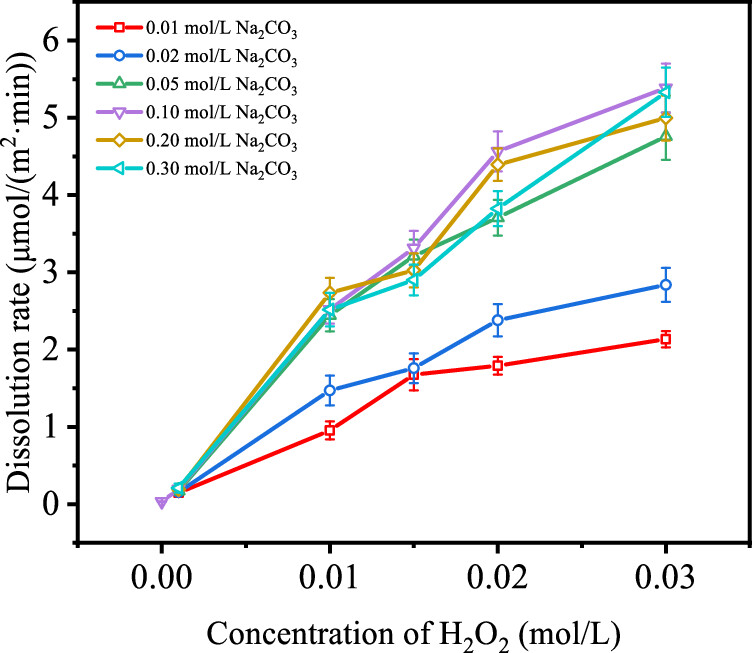
The caption:
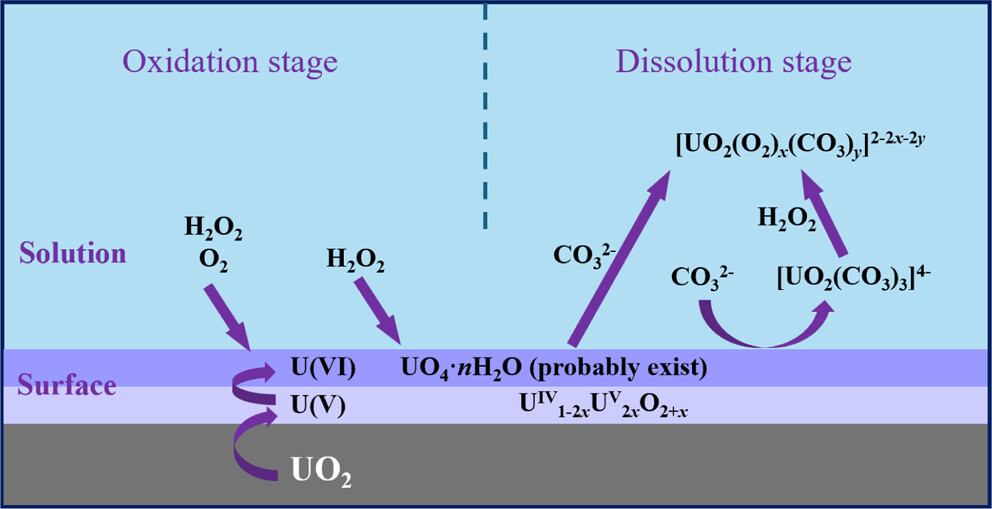
The process takes place in two stages, oxidation followed by dissolution as shown in this cartoon (which also appears in the abstract.)
The caption:
The reactions associated with dissolution:
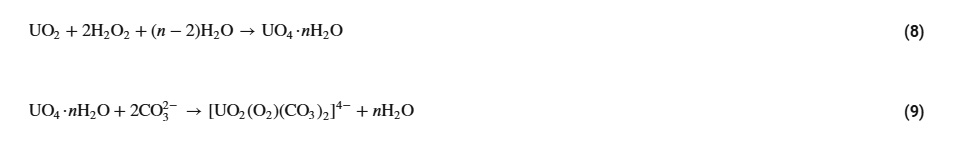
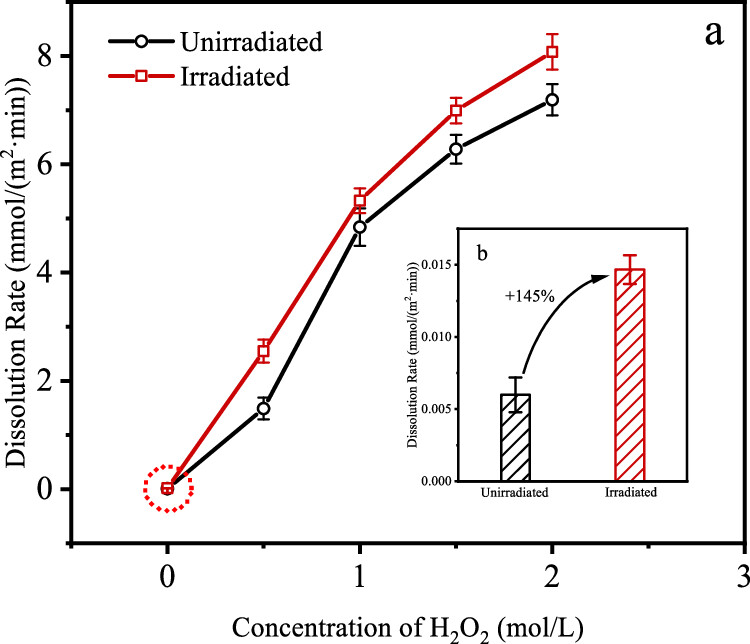
Comparison of the effect of concentration of hydrogen peroxide in irradiation and nonirradation conditions:
The caption:
The Gray is a derived unit of absorbed energy and has units of Joules/kg. It represents the amount of energy deposited in mass by radiation.
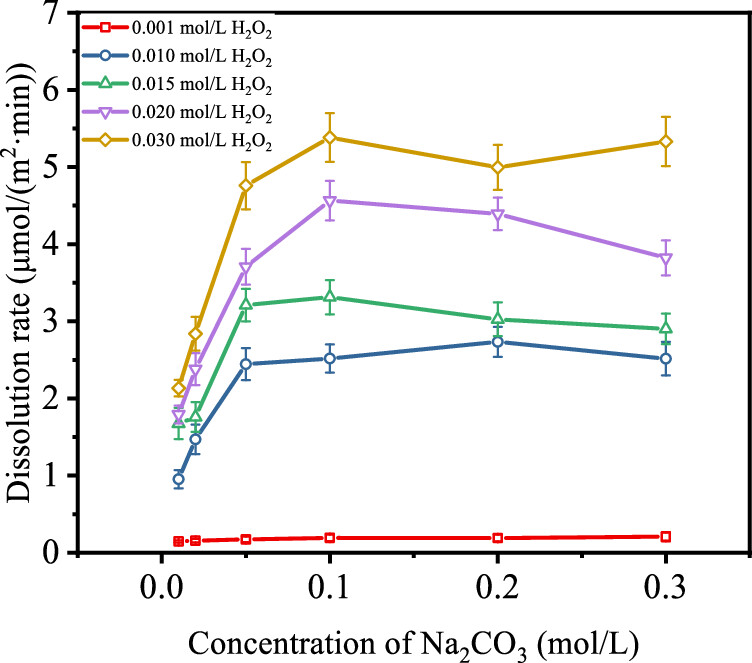
The effect of carbonate concentrations under rradiation and nonirradation conditions. (Note that there is a maxima.)
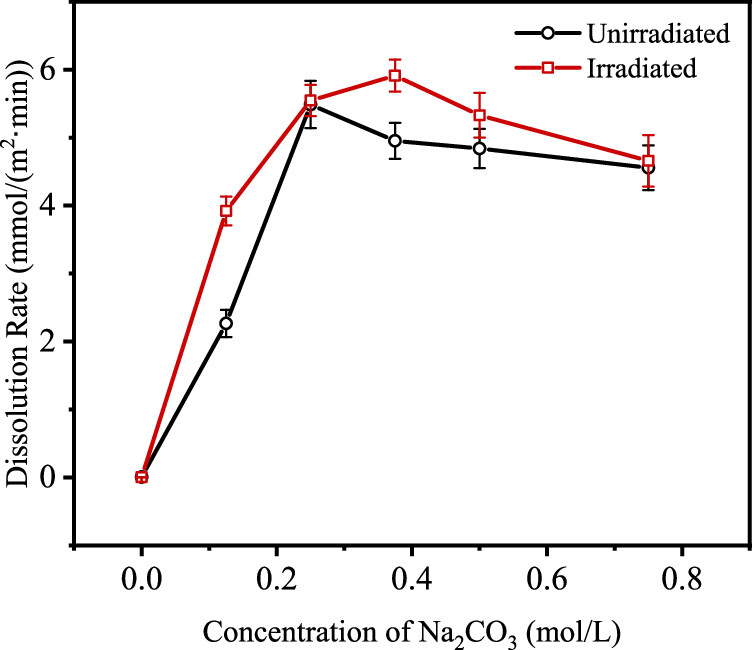
The caption:
The more absorbed radiation, the better:
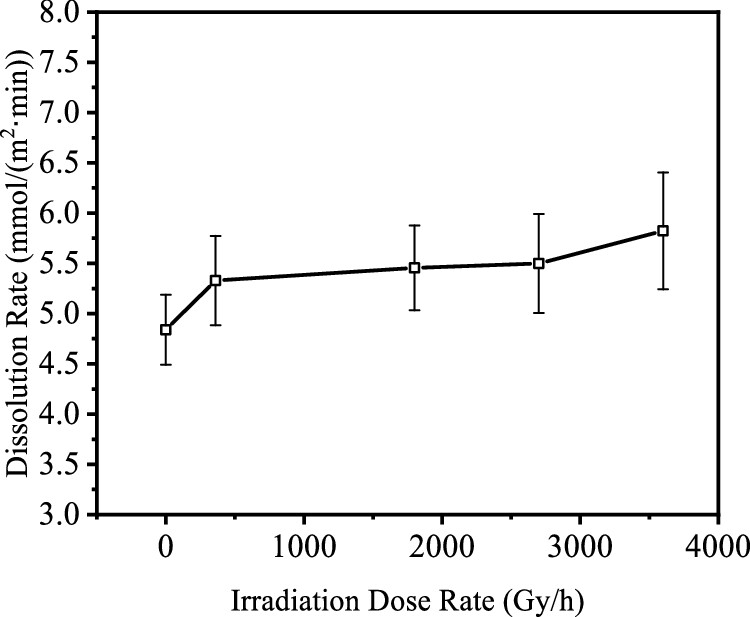
The caption:
Radiation increases the dissolved uranium:
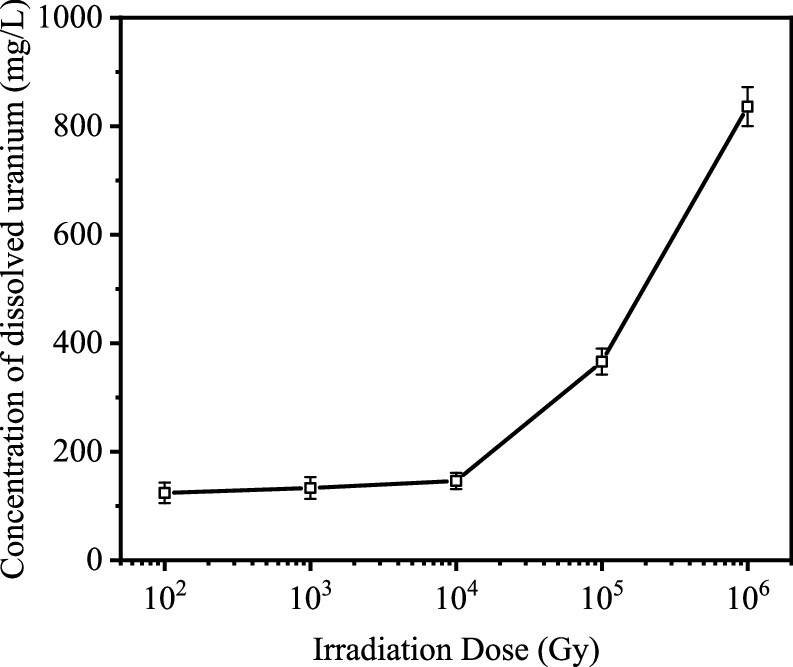
The caption:
The authors note that the experimental conditions are very different than might take place in a putative so called "nuclear waste" dump, if, in a persistent but dubious "dump" mentality continues to exist for used nuclear fuel, particularly in the absence of oxidative peroxide:
I enjoyed the paper, especially because it's one in which I learned things I did not know.
Conceivably, a better world is possible, but, under the circumstances, unlikely. Respecting the value of used nuclear fuel, seeing as a gift to future generations rather than another liability in the plethora of other liabilities, far more dire, the worst being the collapse of the planetary atmosphere, would be an honorable idea.
However honor, as a concept, is becoming irrelevant, it seems. History will not forgive us, nor should it.
Have a nice weekend in spite of it all.